
PRO/II Process Simulation
Gibbs Reactor
for
Bio Gasification
by Greenwood
![]()
 |
PRO/II Process SimulationGibbs ReactorforBio Gasificationby Greenwood |
|
Introduction
The purpose of this study is the validation of home made simulation program "Power Generation by Biomass Gasification Version 3".
Input
Same operating condition were used.
Biomass Feed
Temp. ::500, 600, 700, 800, 900 oC
Pressure:1atm
|
kgmol/h as elements |
kgmol/h as molecule |
|
| Carbon as Solid | 0.04408 | 0.04408 |
| Hydrogen | 0.06906 | 0.03453 |
| Oxygen | 0.03380 | 0.01690 |
| Water | 0.02834 | 0.02834 |
Air feed
Temp. :500, 600, 700, 800, 900 oC
Pressure:1atm
|
kgmol/h as molecule |
|
| Oxygen | 0.01400 |
| Nitrogen | 0.05267 |
Gibbs Reactor
Temp. :500, 600, 700, 800, 900 oC
Pressure:1atm
Results
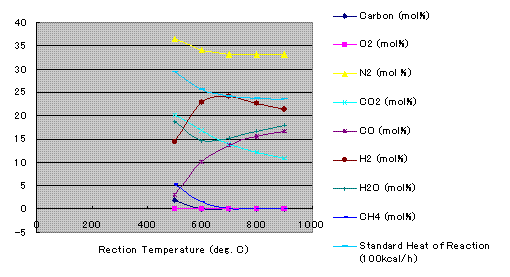
Coments
PRO/II uses Newton Raphson or Marquart Method for finding minimum Gibbs free energy. It assumes that minimum point was achieved when 1st order derivative of G becomes zero. For this purpose, 2nd order derivatives are also calculated. Whereas, Solver use numerical differential coefficient.
In this validation, standard heat of formation is assumed exactly same as that of elements constituting the biomass. This means that heat of formation of dry biomass DH0fdry is zero. But when you have a data of actual heat of combustion of the biomass, then you can calculate.
DH0fdry=(heat of combustion calculated from elements) - (measured heat of combustion of the biomass)
As
Heat of combustion calculated from elements=0
Then
DH0fdry= - measured heat of combustion of the biomass
You can use this for Gibbs Free Energy calculation and for heat balance.
January 23, 2010